Introduction
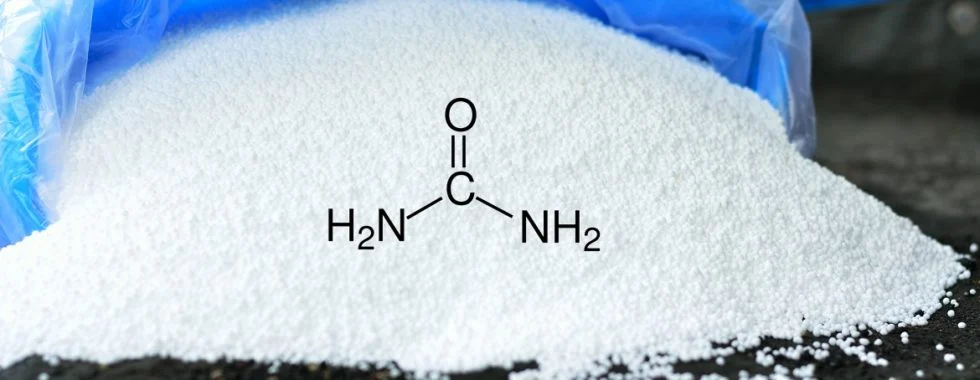
Urea (chemical formula: CO(NH₂)₂) is one of the most widely used nitrogen-based fertilizers in the world. As a synthetic organic compound, urea plays a crucial role in modern agriculture by providing an essential nutrient—nitrogen—to crops. Its high nitrogen content (approximately 46% by weight) makes it an efficient and cost-effective choice for farmers globally.
This article explores the chemical properties of urea, its production process, application methods, benefits, and environmental considerations.
1. Chemical Properties and Composition
Urea is a white, crystalline solid that is highly soluble in water and non-toxic in nature. It is an organic compound that contains two amine groups (-NH₂) joined by a carbonyl group (C=O).
Molecular Formula: CO(NH₂)₂
Molar Mass: 60.06 g/mol
Nitrogen Content: ~46%
Due to its high nitrogen concentration, it is highly efficient for use in fertilizers, requiring less volume compared to other nitrogen sources.
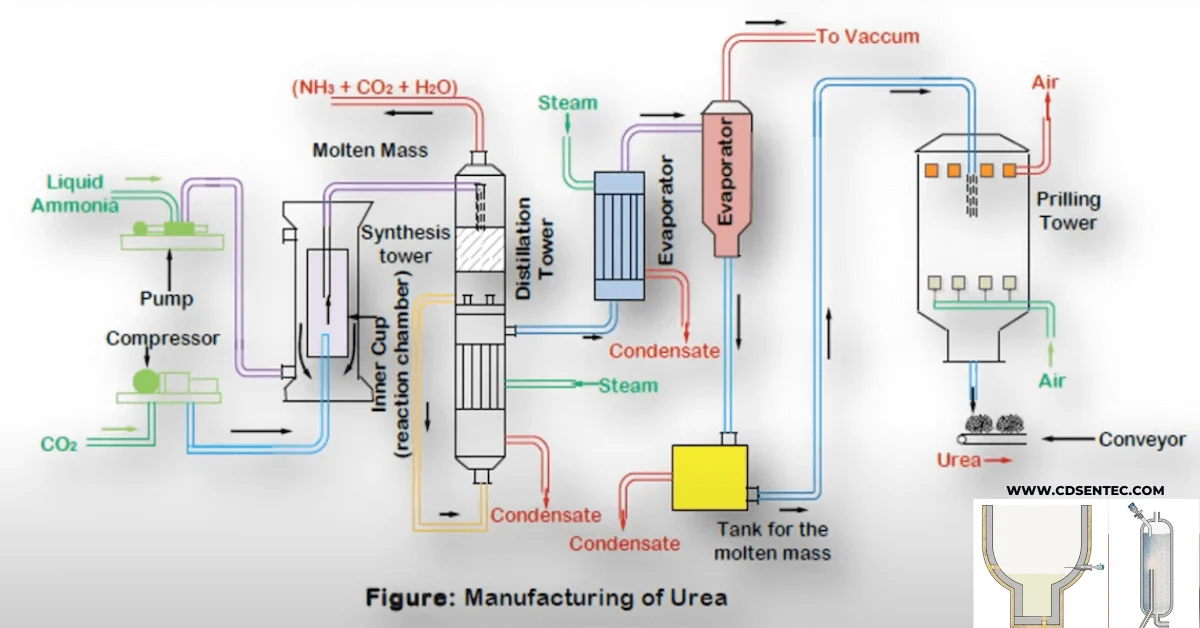
2. Production of Urea
Urea is industrially synthesized through the Bosch-Meiser urea process, which involves two main reactants:
Ammonia (NH₃)
Carbon dioxide (CO₂)
These chemicals are subjected to high pressure and temperature to form ammonium carbamate, which is then dehydrated to produce urea and water.
Reaction:
2 NH₃ + CO₂ → NH₂CONH₂ + H₂O
This process is usually integrated with ammonia production facilities for efficiency and cost-effectiveness.
3. Agricultural Applications
Urea is primarily used as a nitrogen-release fertilizer. Its applications span across various crop types including cereals (wheat, rice, corn), fruits, vegetables, and even grasslands.
Methods of Application:
1. Broadcasting: Spreading urea granules across the soil surface.
2. Top Dressing: Applying urea around the base of crops during the growing season.
3. Foliar Spray: Dissolving urea in water and spraying directly on plant leaves.
4. Fertigation: Applying urea through irrigation systems.

4. Advantages of Urea Fertilizer
High Nitrogen Content: One of the most concentrated nitrogen fertilizers available.
Cost-Effective: Relatively inexpensive to produce and transport.
Non-toxic: Safe for handling and storage when proper precautions are taken.
Versatile: Suitable for a wide range of crops and soils.
5. Environmental Concerns
Despite its agricultural benefits, improper use of urea can cause environmental issues:
A. Nitrogen Loss
Urea can easily volatilize into the atmosphere as ammonia gas, especially when applied on the soil surface without incorporation.
B. Water Pollution
Excess urea can leach into groundwater as nitrate, contributing to eutrophication and contamination of drinking water.
C. Greenhouse Gas Emissions
During microbial processes like nitrification and denitrification, nitrous oxide (N₂O)—a potent greenhouse gas—can be released.
6. Best Practices for Urea Use
To maximize efficiency and minimize environmental damage, farmers can follow these practices:
Use of Urease Inhibitors: Slows down the conversion of urea to ammonia, reducing volatilization losses.
Soil Incorporation: Mixing urea into the soil prevents nitrogen loss to the atmosphere.
Split Application: Applying urea in smaller doses throughout the growing season ensures better nitrogen uptake.
Precision Agriculture: Using soil testing and GPS-guided equipment to apply the right amount of fertilizer at the right time.

7. Conclusion
Urea remains a cornerstone of modern agriculture due to its high nitrogen content, affordability, and adaptability. However, its environmental implications highlight the importance of responsible usage and sustainable farming practices. With proper management, urea can continue to support global food production without compromising environmental health.




